The Electronegativity of Nonmetals Is Relatively
Therefore electron affinity decreases. F Fluorine is the most electronegative element.

Lesson Explainer Electronegativity Nagwa
To easily satisfy the octet rule the nonmetal will accept an electron donated by the metal.
. More than one electron can be donated and received in an ionic bond. Metals have few valence electrons whereas nonmetals have closer to eight valence electrons. Which one of the following bonds has the least ionic.
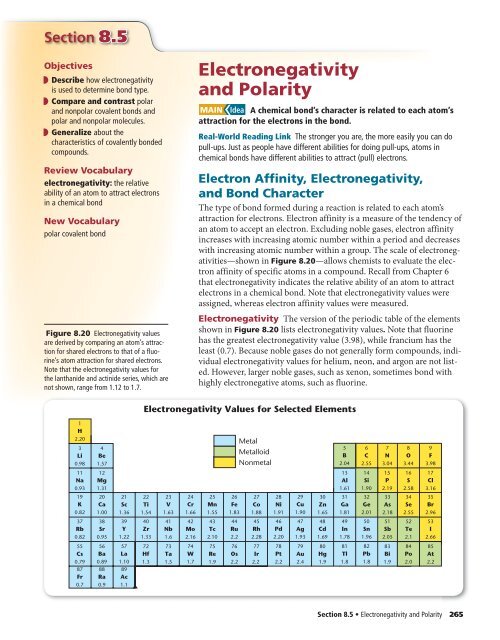
Noble gases - Purple and blue. The higher the associated electronegativity number the more an element or compound attracts electrons towards it. A C B N C O D F.
This is a list of the nonmetal elements in order of increasing atomic number. List of chemical elements. The noble gases are helium neon argon krypton xenon radon and oganesson.
The noble gases are relatively nonreactive gases found in group 8 the last column of the period table. The image shows two atoms surrounded by their electron cloud. The tendency of an atom or.
Moving from left to right across a period atoms become smaller as the forces of attraction become stronger. Choose all that apply - Na tends to donate its outer shell electron to. A nonmetal is a chemical element having among other properties a relatively low density and moderate to high electronegativityBroadly they lack a preponderance of more metallic attributes such as luster deformability good thermal and electrical conductivity and low electronegativity.
Based on the image which of the following are true. It is a member of the chalcogen group on the periodic. Actinide and lanthinide series.
The electron affinity of an element is the energy change which accompanies the addition of an electron to an atom in the gas phase to produce a negatively charged anion. Acts as an electron acceptor and a metal which acts as an electron donor. Its likely oganesson is not a gas at room temperature.
Which one of the following is the most electronegative atom. Polar bonds occur between two nonmetals and have a difference in electronegativities of the atomsThe further apart two atoms are in the same period the greater the polarity of the bond. Nonmetals - Tan.
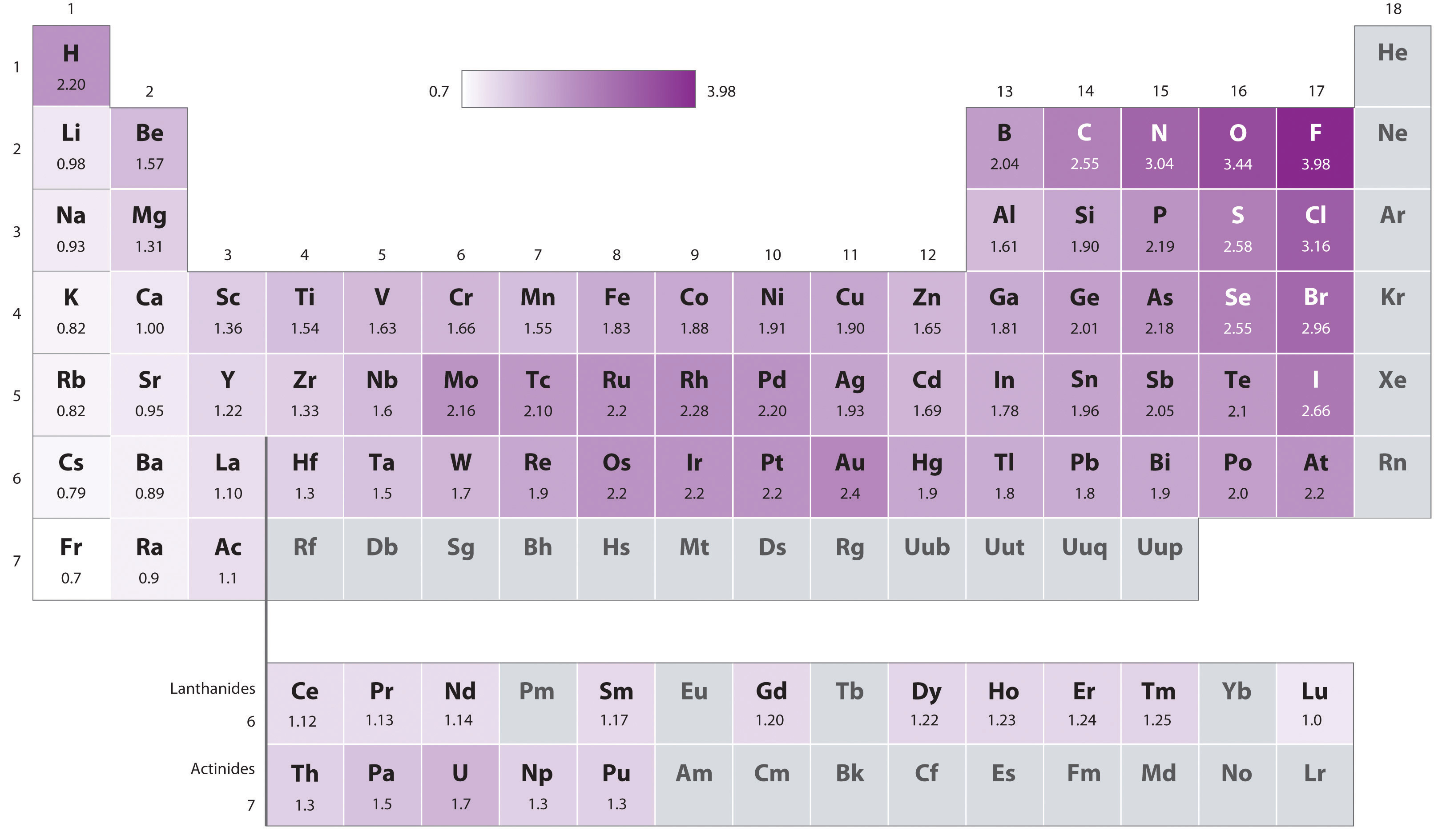
Electron Affinities reported in unites of kilojoules per mole kJmol. The higher the associated electronegativity number the more an element or compound attracts electrons towards it. Data taken from John Emsley The Elements 3rd editionOxford.
In general an atoms electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. In general an atoms electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. With a larger distance between the negatively-charged electron and the positively-charged nucleus the force of attraction is relatively weaker.
Since there is no rigorous definition of a nonmetal some variation may be encountered among. The chemical symbol for Oxygen is O. Oxygen is a colourless odourless reactive gas the chemical element of atomic number 8 and the life-supporting component of the air.
Oxygen is a chemical element with atomic number 8 which means there are 8 protons and 8 electrons in the atomic structure. This causes the electron to move closer to the nucleus thus. Major minor and trans-actinides.
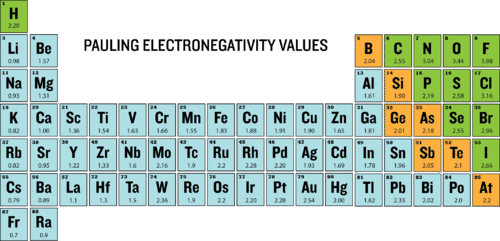
Magnetite and hematite occur together in layered sedimentary rocks called _____. The most electronegative atom fluorine is assigned a value of 40 and values range down to. Xg e- X-g.
The most electronegative atom fluorine is assigned a value of 40 and values.

Electronegativity Table Easy Hard Science
Properties Of Nonmetals And Metalloids By Group Wikiwand

Lesson Explainer Electronegativity Nagwa

Electronegativity Examples Trends Video Lesson Transcript Study Com
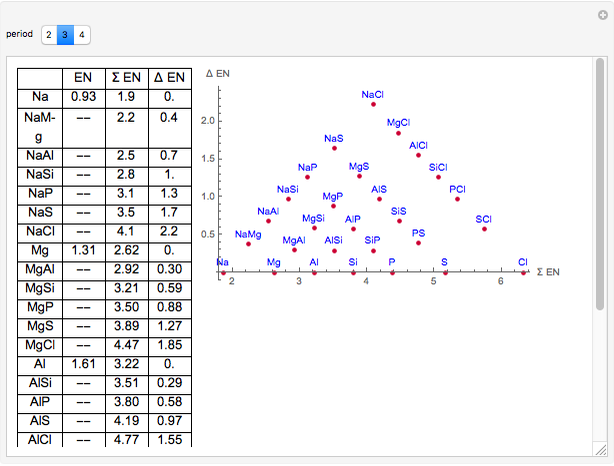
2 12 Electronegativity Chemistry Libretexts

Electronegativity And Bonding Type Wolfram Demonstrations Project
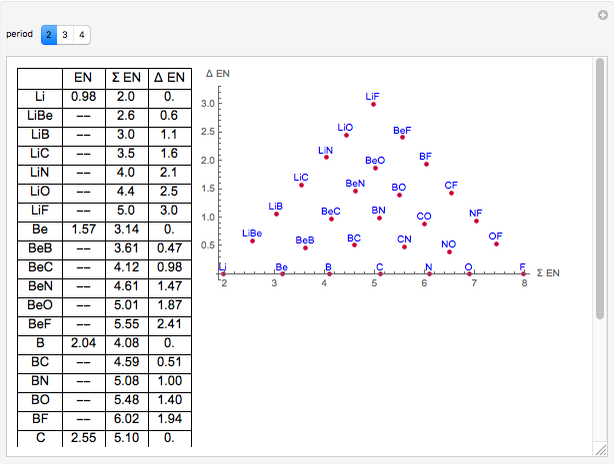
Electronegativity And Bonding Type Wolfram Demonstrations Project
12 2 Electronegativity Chemistrysaanguyen
12 2 Electronegativity Chemistrysaanguyen
Why Are Nonmetals Highly Electronegative Quora

Electronegativity An Overview Sciencedirect Topics

5 Fundamental Properties Of Nonmetals Science Trends
Periodic Trends Electronegativity Ck 12 Foundation

If P Q R And S Are Elements Of 3rd Period Of P Block In Modern

Electronegativity Table Easy Hard Science


Comments
Post a Comment